Why do solids form? The Hamiltonian of the electrons and ions is:
It is invariant under the symmetry. However, the energycan usually be minimized by forming a crystal. At low enough temperature, this willwin out over any possible entropy gain in a competing state, so crystallization willoccur. Why is the crystalline state energetically favorable? This depends on the typeof crystal. Di erent types of crystals minimize diferent terms in the Hamiltonian.In molecular and ionic crystals, the potential energy is minimized. In a molecular crystal, such as solid nitrogen, there is a van der Waals attraction between molecules caused by polarization of one by the other. The van der Waals attraction is balancedby short-range repulsion, thereby leading to a crystalline ground state. In an ionic crystal, such as NaCl, the electrostatic energy of the ions is minimized (one mustbe careful to take into account charge neutrality, without which the electrostatic energy diverges). In covalent and metallic crystals, crystallization is driven by the minimization of the electronic kinetic energy. In a metal, such as sodium, the kinetic energy of the electrons is lowered by their ability to move throughout the metal. Ina covalent solid, such as diamond, the same is true. The kinetic energy gain is highenough that such a bond can even occur between just two molecules (as in organicchemistry). The energetic gain of a solid is called the cohesive energy.
Phonons: Linear Chain
Quantum Mechanics of a Linear Chain
As a toy model of a solid, let us consider a linear chain of masses m connected bysprings with spring constant B. Suppose that the equilibrium spacing between themasses is a. The equilibrium positions de ne a 1D lattice. The lattice `vectors', Rj ,are de ned by:
Rj = ja
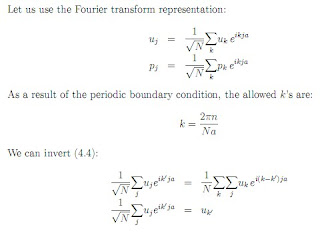
They connect the origin to all points of the lattice. If R and R* are lattice vectors, then R + R* are also lattice vectors. A set of basis vectors is a minimal set of vectors which generate the full set of lattice vectors by taking linear combinations of the basis vectors. In our 1D lattice, a is the basis vector. Let ui be the displacement of the ith mass from its equilibrium position and let pi be the corresponding momentum. Let us assume that there are N masses, and let's impose a periodic boundary condition, ui = ui+N. The Hamiltonian for such a system is:
Hence, k= k + (2pi)m/a
Hence, we can restrict attention to n = 0; 1; : : : ;N. The Hamiltonian can be rewritten:
Note that if we take m = M, we recover the previous results, with a ! a=2.This is an example of what is called a lattice with a basis. Not every site on the chain is equivalent. We can think of the chain of 2N masses as a lattice with N sites. Each lattice site has a two-site basis: one of these sites has a mass m and the other has a mass M. Sodium Chloride is a simple subic lattice with a two-site basis: the sodium ions are at the vertices of an FCC lattice and the chlorine ions are displaced from them.



No hay comentarios:
Publicar un comentario